38 fda health claims on food labels
FDA Proposes to Update Definition for "Healthy" Claim on Food Labels The U.S. Food and Drug Administration today issued a proposed rule to update the definition of the nutrient content claim "healthy.". The "healthy" claim can act as a quick signal on food package labels to help empower consumers, including those with lower nutrition knowledge, with information to identify foods that will help them build ... FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages to ... Español. Today, the U.S. Food and Drug Administration proposed updated criteria for when foods can be labeled with the nutrient content claim "healthy" on their packaging.
FDA perspectives on health claims for food labels - PubMed The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims charact …

Fda health claims on food labels
Authorized Health Claims That Meet Significant Scientific Agreement Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products or dietary supplements to show that a food or food component may reduce the ... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims, and structure/function claims. Learn more about these categories from Label Claims for Conventional Foods and Dietary Supplements. FDA encourages that ... FDA proposes updates to 'healthy' claim on food packages | CNN For example, a cereal would need to contain three-quarters of an ounce of whole grains and no more than 1 gram of saturated fat, 230 milligrams of sodium and 2.5 grams of added sugars, the FDA says.
Fda health claims on food labels. FDA Proposes New 'Healthy' Claim on Food Labels FDA Proposes New 'Healthy' Claim on Food Labels. Sept. 28, 2022. Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. Interested parties have about three ... Questions and Answers on Health Claims in Food Labeling | FDA 4. Has the FDA ever revoked an authorized health claim? The FDA has authorized 12 health claims since 1990. On October 31, 2017, the agency issued a proposed rule to revoke the regulation that ... Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Small Entity Compliance Guide on Structure/Function Claims | FDA On January 6, 2000, the Food and Drug Administration (FDA) published a final rule in the Federal Register defining the types of statements that may be used on the label and in the labeling of ...
How to Start a Food Business | FDA - U.S. Food and Drug ... Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332) Label Claims for Conventional Foods and Dietary Supplements The Nutrition Labeling and Education Act of 1990 (NLEA) permits the use of label claims that characterize the level of a nutrient in a food (i.e., nutrient content claims) if they have been authorized by FDA and are made in accordance with FDA's authorizing regulations. Nutrient content claims describe the level of a nutrient in the product ... 5 Understanding Food Labels and Health Claims - Maricopa To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." A qualified claim has supportive evidence, which is NOT ... FDA Proposes to Update Definition for "Healthy" Claim on Food Labels September 28, 2022. The U.S. Food and Drug Administration today issued a proposed rule to update the definition of the nutrient content claim "healthy.". The "healthy" claim can act as a ...
Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ... Questions and Answers on Health Claims in Food Labeling | FDA 4. Has the FDA ever revoked an authorized health claim? The FDA has authorized 12 health claims since 1990. On October 31, 2017, the agency issued a proposed rule to revoke the regulation that authorizes the use of a health claim about the relationship between soy protein and the reduced risk of coronary heart disease. Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA U.S. Food and Drug Administration, Guidance for Industry, Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements July 2003.
Qualified Health Claims | FDA - U.S. Food and Drug Administration Qualified Health Claims. Qualified health claims (QHCs) are supported by scientific evidence, but do not meet the more rigorous "significant scientific agreement" standard required for an authorized health claim. To ensure that these claims are not misleading, they must be accompanied by a disclaimer or other qualifying language to ...
The Legality of Food Labeling Claims: FDA's Regulations First, the FDA can authorize a health claim after it determines there is significant scientific agreement among health experts that the claimed relationship between the nutrient and reduced risk of disease exists. 21 U.S.C. § 343(r)(3)(B). Second, any interested person may petition the FDA to issue a regulation authorizing a health claim. 21 C ...
Fda Health Claims On Food Labels FDA Proposes to Update Definition for "Healthy" Claim on … Health (7 days ago) September 28, 2022. The U.S. Food and Drug Administration today issued a proposed rule to update the definition of the nutrient content claim "healthy.".
Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Use of the Term Healthy on Food Labeling | FDA Nutrient Content Claim "Healthy". Claims like "healthy" on food labels can provide information to consumers to help them identify healthier food choices at a quick glance. Foods must meet ...
Organic on Food Labels | FDA Oct 12, 2022 · The FDA does not regulate the use of the term “organic” on food labels, including dietary supplements. The National Organic Program (NOP) is the federal regulatory framework governing ...
Everything you need to know about Health Claims on Food Labels The "qualified" health claims. The authorized health claims by the FDA must have significant scientific agreement among qualified experts to support the scientific evidence for a substance - disease relationship. The FDA has approved 12 health claims on food labels such as sodium and hypertension; fiber-containing grains, fruits and ...
FDA proposes updates to 'healthy' claim on food packages | CNN For example, a cereal would need to contain three-quarters of an ounce of whole grains and no more than 1 gram of saturated fat, 230 milligrams of sodium and 2.5 grams of added sugars, the FDA says.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims, and structure/function claims. Learn more about these categories from Label Claims for Conventional Foods and Dietary Supplements. FDA encourages that ...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products or dietary supplements to show that a food or food component may reduce the ...




:max_bytes(150000):strip_icc()/juicy-juice-no-sugar-400x400-b1fb04c46e9e4c8392ce8881614c021a.jpg)
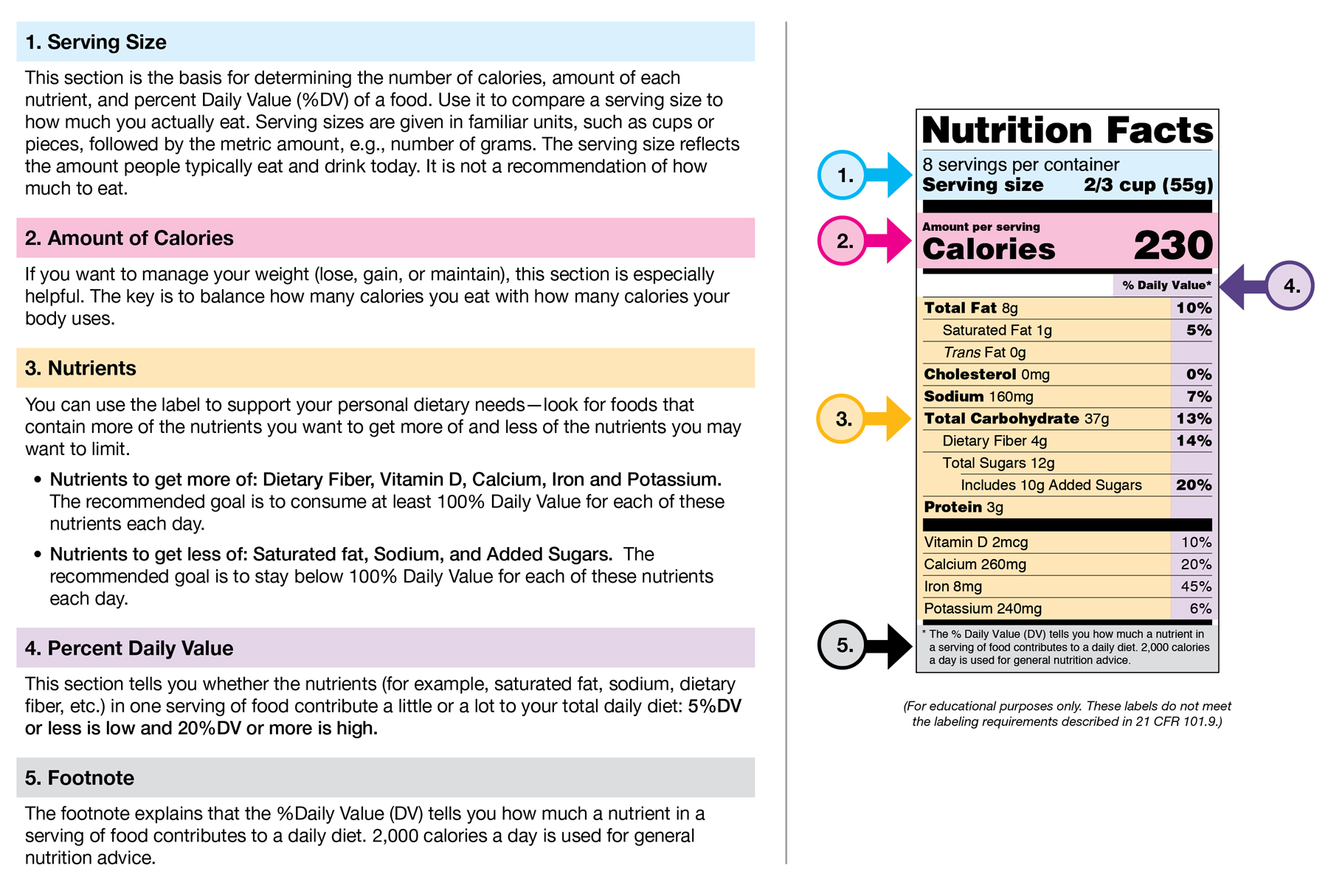











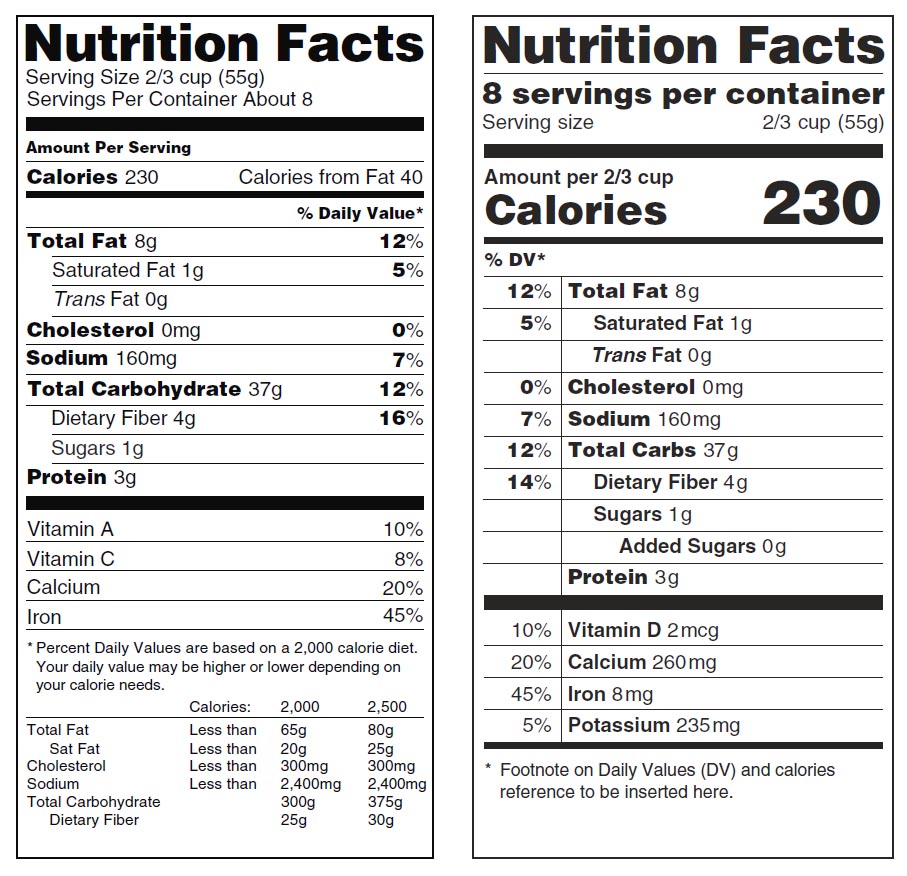













Post a Comment for "38 fda health claims on food labels"